Solvothermal synthesis can be described as wet-chemistry carried out under elevated temperatures and pressures. The Iversen group has an extensive research profile in synthesis of a wide range of functional nanomaterials by exploiting these synthesis conditions.
Read more about the apparatus and how we perform solvothermal synthesis in the lab to generate new nano materials.

Solvothermal synthesis can be performed in batch vessels (autoclaves) or in a flow-system, which is highly convenient for better parameter control, production of larger quantities and industrial aspects. In a flow-reactor, a high-pressure pump actively feeds a solution of dissolved precursor to the system. Another pump feeds pure solvent, which is pre-heated to (typically) 300-450 °C and subsequently mixed with the precursor stream, thus initiating an extremely rapid chemical reaction. This leads to the formation of highly homogeneous nanoparticles which are suspended in the solvent. After mixing, the hot suspension of nanoparticles flows through a heated reactor zone to improve crystallinity. The flow is then cooled and the pressure reduced through a valve.
A variation of this reactor concept is the dual-stage system, where two reactors are serially connected, each with their own mixing piece and a cooling zone in between. The goal is to achieve composite nanoparticles where one material is first synthesized in Reactor #1 and another nanomaterial is subsequently deposited on these primary nanoparticles by means of Reactor #2.
Finally, a slightly different approach is pulsed-flow synthesis, where a continuous chain of small batch volumes are synthesized in a pre-heated, automated reactor. This reactor concept has the advantage of indefinite residence time, which is important in materials where crystallization kinetics are slow.
Between the three concepts, a huge variety of nanoparticle constellations can be produced. Surfactant additives may be co-introduced with precursors in order to yield stable nanodispersions. Similarly, catalyst substrates can be injected in order to synthesize nanoparticles directly on a support material.
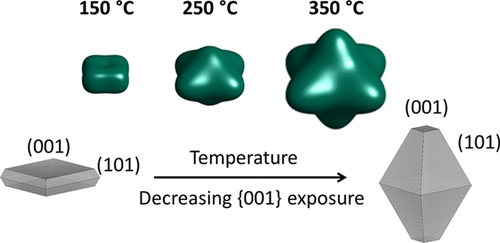
In our article from 2020, a combination of autoclave (ex situ) and in situ powder X-ray diffraction studies were used to optimise the active facets of anatase TiO2 for, e.g., photocatalysis. To the right, the crystallite configuration and facet exposure is represented as a function of temperature, but the reaction time was also found to be of significant importance for optimisation of the {001} facet.
Read more about similar studies on the synthesis and optimisation of nanomaterials in the links below.