Over the last century, the need for fuel-free energy has become clear and thereby the need for being able to store energy. The energy storage industry has continued to evolve and adapt to changing energy requirements and advances in technology but are still far from being able to store large amount of energy for later use.
Energy storage systems provide a wide array of technological approaches to managing power supply. The goal is to bring cost savings to utilities and consumers by creating a more resilient energy infrastructure.
Hydrogen storage is a key enabling technology for the advancement of hydrogen and fuel cell technologies in applications including portable power, stationary, and transportation. Hydrogen is special from all other elements by having the highest energy per mass of any fuel. However, one of the challenges is its low ambient temperature density that results in a low energy per unit volume. When hydrogen is stored in other materials, the energy per unit volume can be improved remarkable.
The material used to store hydrogen must be able to release the hydrogen in a reversible process that allows hydrogen to be absorb again. The reversible property is a key challenge due to produce high-quality hydrogen energy storage facilities.
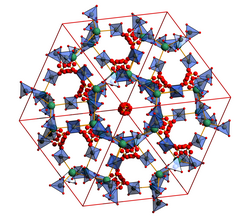
Zeolite like gamma-Mg(BH4)2 possesses large pores capable of hosting small guest molecules such as N2, CH2Cl2 or H2. The extra H2 results in an extreme gravimetric hydrogen content (17.4 wt. %) and upon compression that same material possesses a volumetric hydrogen content of 147 kg/L, second only to Mg2FeH6. Thus, gamma-Mg(BH4)2 displays both chemisorption and physisorption capabilities of hydrogen and as such is a highly attractive material for solid state hydrogen storage.
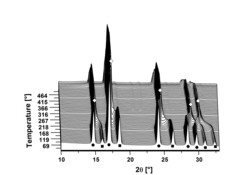
In situ X-ray experiments gives insight in the reactions mechanism of new compounds and the reaction pathway for releasing hydrogen during decomposition. In the figure below the formation of the solid solution Na1-xKxBH4 (ο) is followed from the starting reactants NaBH4 (■) and KBH4 (●) during heating. You can read more about in situ X-ray experiments here.

Do you want to read more about hydrogen storage in Danish? The paper Energiopbevaring - nøglen til en fossilfrit fremtid was published in Aktuel Naturvidenskab in 2014. Read it here.