By changing chemical or physical environments sufficiently, materials may be forced to undergo temporary or meta-stable structural transitions which could not be brought about in any other way. Since atomic structure and macroscopic properties are intimately related, studying both under various “extraordinary” conditions is a source of great insight into structural chemistry and discovery of entirely new materials.
One example from the Iversen group is the property of photo-magnetism, i.e. a material becoming magnetic upon irradiation with light in the visible- or UV-range. The photons induce electronic transitions in the structure, and since electrons are the essence of chemical bonds, these transitions alters the crystal structure in subtle ways which in turn leads to a net magnetic moment. The prospect of magnets which can be switched on and off by a light source is very interesting in itself, but more importantly photo-magnetism provides an opportunity to investigate the features of an atomic structure which give rise to magnetism.
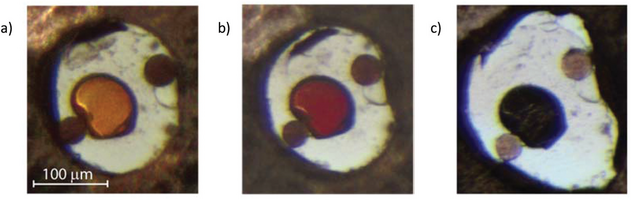
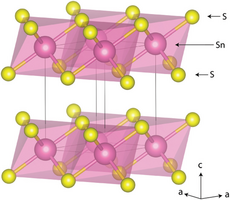
Another example of “extreme conditions” is materials created by means of physical pressure. Ultra-high pressures (>100.000 bar) is a driving force for chemical reactions and structural transitions, which can lead to meta-stable materials which otherwise do not exist on the Earth’s surface. The fundamental reason is that the PV term of the free energy reaches a few electron volts at high pressure, which is the energy scale of the chemical bond. In the Iversen group, we create these conditions by means of diamond anvil cells (DACs) where a single crystal of a material is mounted between two opposing diamonds inside a metal gasket. The DAC may then be mounted on an X-ray diffractometer (in-house or at synchrotrons) where the X-ray beam enters through one of the diamonds, scatters on the sample and exits through the other diamond. The result is a “window” into the structural changes as they happen inside the ultra-high-pressure environment. To the right is the layered atomic structure of SnS2, and the corresponding visible changes in a SnS2 crystal as a function of pressure ((a) 0.66(1) GPa, (b) 8.1(1) GPa, (c) 16.8(4) GPa, respectively).