The electron-density (ED) distribution of a molecular-scale system is arguably the most information-rich observable in natural science.
The ED is a scalar function that shows the distribution of electrons across an entire unit cell of any material, and therefore the full electron distribution throughout the whole structure. Thus, it occupies a key role in chemistry, enabling scientific rationalization in terms of fundamental concepts such as atoms, chemical bonds and functional groups. This kind of understanding relies on the intimate link existing between the physical properties of a material and its ED. It is feasible to determine experimental EDs with accurate high-resolution X-ray diffraction experiments. Some data are collected on in-house diffractometers while very high quality data are acquired at large-scale synchrotron facilities.
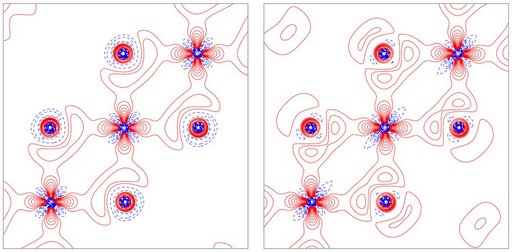
Through the past two decades we have investigated the ED and chemical bonding of a wide range of technologically relevant materials in order to truly comprehend the origin of intriguing features such as magnetism, ion conduction, guest-host interactions and thermoelectricity. For example, we have provided a quantitative description of VdW bonding in layered materials of huge technological importance (2D materials such as TiS2; the static deformation density and Laplacian for the interlayer Ti-S interaction is shown right for the TiS4 plane and the TiS6 octahedron from multipole modelling). It was shown that current DFT theory cannot reproduce highly accurate X-ray diffraction data. The nature of vdW interactions are also important for rubrene, which is the best performing organic semiconductor.
We have also provided a detailed description of stereoactive lone pairs in materials with low thermal conductivity. The expression of a stereo active lone pair provides a special chemical bonding situation, and quantitative understanding of the phenomena allows design of materials with new properties. The thermal conductivity in high performance low cost thermoelectric Mg3Sb2 has a peculiar isotropic nature, which does not conform with the general description of the structure as layered. Detailed analysis of the electron density provided a proper chemical bonding picture with an almost isotropic bonding network.
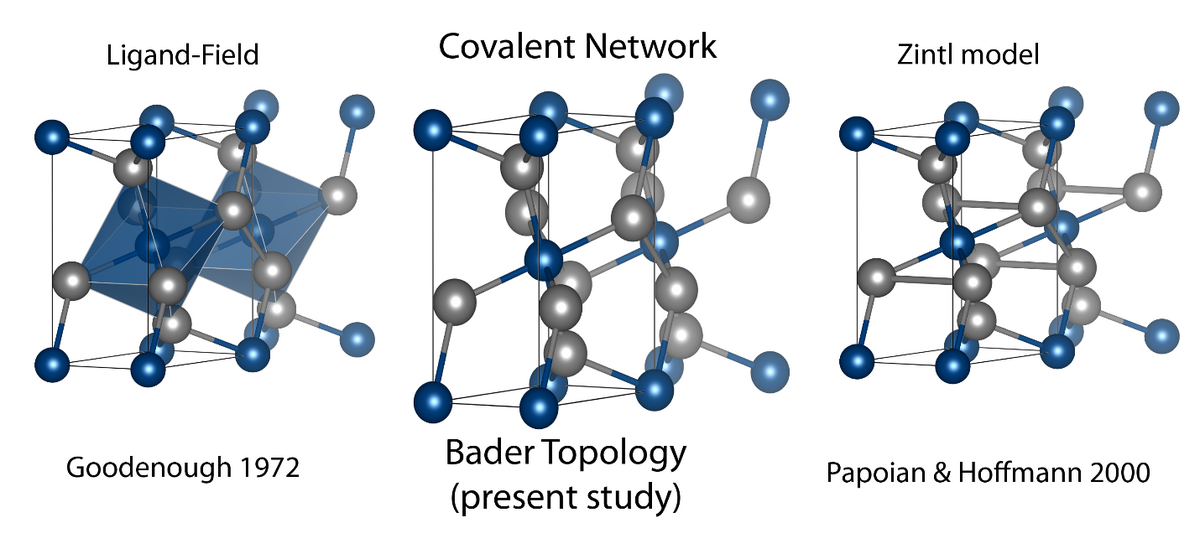
In 2007, we discovered that Marcasite, FeSb2, has the highest known thermoelectric power factor and in fact the chemical bonding in Marcasite structures have been used to test bonding theories for several decades. An experimental charge density does not align with the current main bonding schemes consisting of ligand-field stabilization or Zintl phase descriptions. In fact, our recent work shows that the Fe-Sb bond is covalent.
Experimental EDs have always been determined from single crystal X-ray diffraction data. However, when single crystals of adequate quality are not available it may be possible to use very accurate powder diffraction data as basis for multipole modelling of the ED. Generally, the knowledge offered by ED studies advance our capability to intelligently design new materials possessing a complete package of properties tailored to their intended applications (click here for our recent review article).
Click on the links below to see recent Electron Density studies from the Iversen Group.