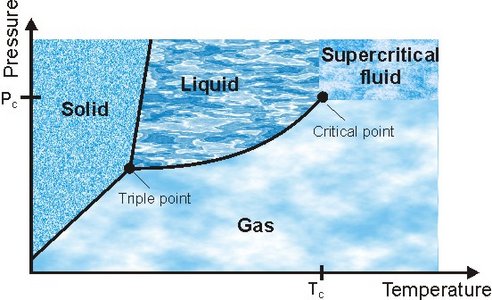
A substance is normally classified by either being solid, liquid or gaseous, however, above a critical point in temperature (TC) and pressure (PC) the substance can no longer be classified as either liquid or gas; it has become a supercritical fluid (SCF). A SCF can diffuse through solids like a gas, and dissolve materials like a liquid. Properties such as the density and polarity can be tuned around the critical point and even small changes in pressure and temperature can change the properties enormously. As an example the ion product of water (Kw) increases three orders of magnitude from ambient towards the critical point, and then just above Tc it drops seven orders of magnitude. Water changes from being a highly polar solvent (e.g. suitable for dissolving ionic solids) to becoming completely non-polar and miscible with organic solvent.