

Interfaces play a deciding role in many aspects of modern chemistry and material science – catalysis, adhesion, sensing, nucleation are all processes driven by interfaces.
We use methods based on static and time-resolved sum frequency generation to probe the orientation, structure and dynamics of molecules at interfaces. Near-edge X-ray absorption fine structure (NEXAFS) spectroscopy and microscopy are used as complementary tools to probe binding chemistry, surface distribution and molecular structure.
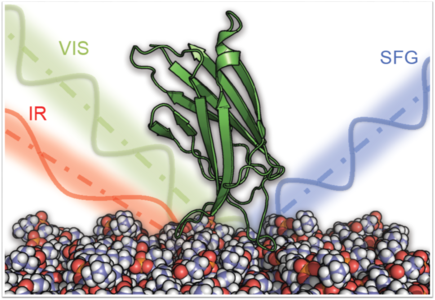
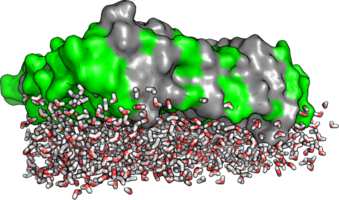
An important part of our research are protein structures at interfaces. Specific proteins can act as Nature’s engineers of both hard and soft tissue. Proteins can ‘sculpture’ biogenic minerals and shape cell membranes. The control interfacial proteins exert over biological surfaces has relevance for disciplines as diverse as cell biology, bio-sensor research, biomimetics and material science. We ask how proteins fold and move at surfaces and how energy flows through protein interfaces.
For technical applications we use chemical modification of surfaces to prevent biofouling and scaling and to reduce friction. The approaches we use are inspired by our studies of the surface chemistry of animals. Can we fabricate self-cleaning surfaces like plants? Stick to walls like a spider? Glue like a frog tongue?
The goal of our research is to understand how molecules operate at surfaces and how we can control interfacial processes at the molecular level.


October 2024
Surflab within the Department of Chemistry at Aarhus University is looking for two postdocs
Postdoc for spectroscopy of biocatalytic surfaces (Deadline 1st of October)
Will follow how enzymes can break down toxic molecules at catalytic surfaces using molecular spectroscopy.
Postdoc for probing chemistry at atmospheric interfaces
(Deadline 16th of October)
Will concentrate on the study of molecular structure at the surface of aerosols and water droplets.

September 2024
Surflab presented a poster at Matchmaking '24 for interested bachelor students seeking projects. See the open projects for more details and contact us if you are interested or have further questions.

August 2024
PostDoc, Kris Strunge won a prize for his poster at the Gordon Research Conference on Vibrational Spectroscopy entitled "Sum Frequency Spectroscopy and Simulations of Interfacial Protein and Water Structure"!
Congratulations Kris!

August 2024
SurfLab researchers, in collaboration with the Biomodelling group at AU and Novozymes, have published a paper in Journal of Physical Chemistry B, entitled “Investigating Lipase/Stain Interactions: Determining Interfacial Protein Conformation with Surface Spectroscopy”.
This study uses vibrational sum frequency generation spectroscopy to directly probe the conformational changes and interactions of a laundry detergent lipase at hydrophobic interfaces, providing insights into protein structure and function relevant to enzyme design and drug discovery.

Monday the 17th of June 2024 at 14.00 in Aud I (1514-213)
Kris Strunge is defending his PhD thesis ”Modelling Vibrational Sum Frequency Generation Spectra of Interfacial Proteins”.

May 2024
SurfLab researchers, together with Thomas la Cour Jansen from the University of Groningen, have published a paper in PCCP entitled “The secondary structure of diatom silaffin peptide R5 determined by two-dimensional infrared spectroscopy”.
Diatoms, unicellular marine organisms, efficiently produce silica nanostructures for their shell walls under ambient conditions, outperforming industrial methods. This process has become highly sought after by materials scientists aiming to develop biomimetic applications. Diatoms achieve this by utilizing short peptide repeats, like the R5 peptide, from silaffin proteins to construct their silica shells. In this study, we employed two-dimensional infrared spectroscopy (2DIR) to determine the secondary structure of the R5 peptide during biosilicification, enhancing our understanding of its role in the process.
The paper was highlighted on the cover of PCCP, showcasing a watercolor painting of diatoms by Inge Heise Thomassen.