

Interfaces play a deciding role in many aspects of modern chemistry and material science – catalysis, adhesion, sensing, nucleation are all processes driven by interfaces.
We use methods based on static and time-resolved sum frequency generation to probe the orientation, structure and dynamics of molecules at interfaces. Near-edge X-ray absorption fine structure (NEXAFS) spectroscopy and microscopy are used as complementary tools to probe binding chemistry, surface distribution and molecular structure.
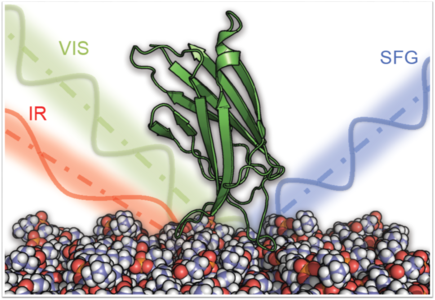
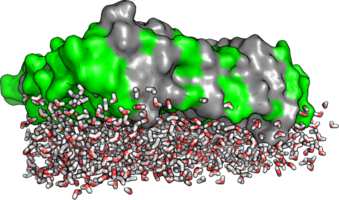
An important part of our research are protein structures at interfaces. Specific proteins can act as Nature’s engineers of both hard and soft tissue. Proteins can ‘sculpture’ biogenic minerals and shape cell membranes. The control interfacial proteins exert over biological surfaces has relevance for disciplines as diverse as cell biology, bio-sensor research, biomimetics and material science. We ask how proteins fold and move at surfaces and how energy flows through protein interfaces.
For technical applications we use chemical modification of surfaces to prevent biofouling and scaling and to reduce friction. The approaches we use are inspired by our studies of the surface chemistry of animals. Can we fabricate self-cleaning surfaces like plants? Stick to walls like a spider? Glue like a frog tongue?
The goal of our research is to understand how molecules operate at surfaces and how we can control interfacial processes at the molecular level.

February 2026
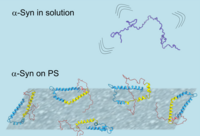
Our paper titled "The Structure of Alpha-Synuclein Bound to Polystyrene Surfaces Probed by Experimental and Theoretical Sum Frequency Generation Spectroscopy" is accepted in Langmuir. In this work, we have determined the surface structure of alpha synuclein at physiological concentration using a combination of vibrational sum-frequency generation spectroscopy and molecular-dynamics simulations. Our results show that alpha synuclein folds in a well-defined helical conformation where the directly bound regions to the polystyrene surface are linked with protein aggregation.

February 2026
Emma Elvira Tranberg Christiansen started as a bachelor student in our group. She will be investigating the chemical and physical changes to plastic in the ocean. Welcome Emma!

January 2026
We held our annual SurfLab Christmas lunch at the Træskibshavn Club House, including a talk from SurfLab alumn Kris Strunge (now at Hovedstadens Beredskab), a traditional Danish Christmas Lunch, a great musical performance by group members and the spectacular Feuerzangenbowle finale!

December 2025
SurfLab went for an excursion to Moesgaard to investigate what hidden questions we can answer with our techniques. This fun outing ended with passionate scientific discussions, coffee and cake !

December 2025
Gayatri joined the group as a post doc and she plans to investigate atmospheric aging and reactivity of airborne nanoplastics using sum frequency generation spectroscopy. Welcome Gayatri !
October 2025
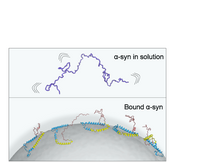
Our paper on "Pathological Folding of α-Synuclein on Polystyrene Nanoplastic Revealed by Sum Frequency Scattering and 2D Infrared Spectroscopy" is accepted in JPC letters. This study found that human alpha synuclein misfolds on polystyrene nanoplastics. Understanding the interfacial structure of alpha synuclein on nanoplastics is crucial to access the role of nanoplastics in the formation of toxic aggregates linked to neurological disorders.
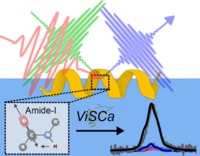
October 2025
Our tutorial paper titled “ViSCa: A computational toolbox for vibrational spectral calculations—Application to sum frequency generation spectroscopy of proteins” was recently published in the Journal of Chemical Physics. This tutorial introduces the Vibrational Spectra Calculation (ViSCa) toolbox, a computational suite of methods for vibrational sum frequency generation (VSFG) spectral calculations of proteins at interfaces. All of the code is also made publicly available at our GitHub page. Included are several illustrative examples that showcase the different functionalities of ViSCa, including determining the orientation of a known protein structure at an interface (“ViSCa-Orient”), as well as coupling the calculations to molecular dynamics simulations to determine distinct changes in protein structure driven by the interfacial environment (“ViSCa-Select”).

September 2025
Pelle Roos Hansen continues in the group for his Master's thesis. He will be working on using 2DIR spectroscopy to study ice nucleating proteins. Welcome back Pelle!
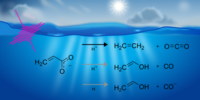
September 2025
Our paper titled "The Primary Photolysis of Acrylate" has been accepted in PCCP. This study uncovers the primary photoproducts of aqueous acrylate by identifying distinct dissociation channels leading to CO2, ethene, and acetaldehyde. Acrylate photodissociation is important to understand secondary organic aerosol formation and its photochemical cycling in the environment.

September 2025
SurfLab presented a poster at Matchmaking '25 for interested bachelor students seeking projects. See the open projects for more details and contact us if you are interested or have further questions.

August 2025
Ásmundur Smári Ragnarsson joins us as a PhD student after completing his Master’s thesis in the group. Welcome Ásmundur!

July 2025
Our paper in ACS Nano Letters titled “Polystyrene Nanoplastic Contaminants Denature Human Apolipoprotein A-1” has been accepted. This work explores how environmentally prevalent nanoplastics interact with proteins found in our body. Studying these interactions is extremely important for determining the possible consequences that ingestion of nanoplastics may have for our health.

Jun 2025
Ásmundur Smári Ragnarsson successfully defended his Master's thesis titled "Fibrinogen Orientation at the Surface of Polydimethylsiloxane and Polystyrene Nanoparticles". Congrats Ásmundur!
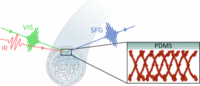
May 2025
SurfLab researchers, in collaboration with Caitlin Howell at the University of Maine, have published a paper in Langmuir, entitled “The Orientation of Human Fibrinogen at Biomedically Relevant Polydimethylsiloxane–Water Interfaces”. This study investigates the causes of catheter-associated urinary tract infections (CAUTIs) by examining the binding of human fibrinogen to PDMS surfaces using vibrational sum frequency generation spectroscopy and structural modeling.

March 2025
Monika Chaudhary has started in the group as a postdoc, who will be following how enzymes can break down toxic molecules at catalytic surfaces using molecular spectroscopy. Welcome Monika!

February 2025
Cesare Meroni has started in the group as a postdoc, who will be working on applying sum frequency scattering spectroscopy to study the molecular structure of the surface of aerosols. Welcome Cesare!

February 2025
SurfLab researchers in collaboration with Stanislav N. Gorb (Kiel University) and Agnieszka Kreitschitz (University of Wrocław), have published a paper in Journal of Soft Matters entitled “Adhesion of the mucilage envelope of Ocimum basilicum seeds probed by sum frequency generation spectroscopy”. Here, we investigate the adhesion mechanism and the adhesion strength of the mucilage envelope at two different substrates. The paper was highlighted on the cover of Soft Matter.

January 2025
SurfLab researchers in collaboration with Jacopo Catalano (AU) and Christian Husum Frederiksen (DTU Offshore), have published a paper in Beilstein Journal of nanotechnology entitled “Bioinspired nanofilament coatings for scale reduction on steel”. The work focused on characterization of the coating for stainless steel and results showed a 75.5% reduction of calcium carbonate deposition on the steel surfaces.