Our group applies quantum chemical and machine learning methods to understand atmospheric processes at the molecular level. This ranges all the way from understanding how individual volatile molecules are oxidized in the atmosphere to cluster formation and growth of formed low volatile species.
Below are outlined some of our core research interests in the group with related recent publications.
The formation of atmospheric clusters and their early growth into stable aerosol particles comprises the largest uncertainty in global climate estimation. We study how clusters are formed in the atmosphere and especially try to elucidate which compounds might be important in the process. Using a combination of quantum chemical calculations and kinetics modelling, we can directly follow the growth mechanism of how aerosol particles are formed.
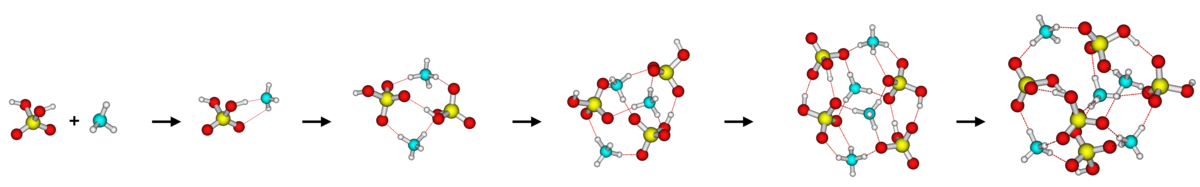
Recent publications:
M. Engsvang and J. Elm, ACS Omega, 2025, 10, 23, 24887–24896.
M. Engsvang and J. Elm, ACS Omega, 2024, 9, 29, 31521–31532.
A. N. Pedersen, Y. Knattrup, and J. Elm, Aerosol Res., 2024, 2, 123–134.
M. Engsvang, Y. Knattrup, J. Kubečka, and J. Elm, Environ. Sci. Technol. Lett., 2024, 11, 101–105.
Y. Knattrup, J. Kubečka and J. Elm, J. Phys. Chem. A 2023, 127, 36, 7568–7578.
Y. Liu, H-B. Xie, F. Ma, J. Chen and J. Elm, Environ. Sci. Technol. 2022, 56, 12, 7751-7760.
M. Engsvang and J. Elm, ACS Omega, 2022, 7, 9, 8077-8083.
R. Zhang, J. Shen, H.-B. Xie, J. Chen and J. Elm, Atmos. Chem. Phys., 2022, 22, 4, 2639-2650.
H.-B. Xie and J. Elm, Atmosphere, 2021, 12, 10, 1260-1271.
Clusteromics:
Y. Knattrup, J. Kubečka, D. Ayoubi and J. Elm, ACS Omega, 2023, 8, 28, 25155–25164.
D. Ayoubi, Y. Knattrup and J. Elm, ACS Omega 2023, 8, 10, 9621–9629.
Y. Knattrup and J. Elm, ACS Omega 2022, 7, 35, 31551-31560.
J. Elm, ACS Omega, 2022, 7, 17, 15206-15214.
J. Elm, ACS Omega, 2021, 6, 26, 17035-17044.
J. Elm, ACS Omega, 2021, 6, 11, 7804-7814.
Review papers:
We utilize quantum machine learning (QML) techniques to boost our quantum chemical calculations. This is currently a work in progress and we are attempting to apply QML to cluster formation studies.
Recent publications:
M. Engsvang, J. Kubečka and J. Elm, ACS Omega, 2023, 8, 34597–34609.
Y. Knattrup, J. Kubečka, D. Ayoubi and J. Elm, ACS Omega, 2023, 8, 28, 25155–25164.
There persist a gap between the clusters being modelled using quantum chemical methods (~1 nm or below) and particles being measured in the lab and the field (~2 nm and above). The modelling of Freshly Nucleated Particles (FNPs) in this transition regime is one of our emerging research topics.
We apply molecular dynamics (MD) simulations to study atmospheric processes. For instance, collisions between molecules and clusters can be studied using steered MD and evaporation free energies can be simulated via umbrella sampling (US).
A variety of volatile organic compounds (VOCs) are continuously emitted into the atmosphere. For instance, there is a large emission of compounds from vegetation (isoprene, alpha-pinene and limonene) and antropogenic sources (aromatics). VOCs are oxidized in the troposphere by chemical reactions with O3 and OH/NO3 radicals. The reactions leads to organic compounds with higher oxygen content which tend to be lower in volatility and more readily can partition to the particle phase.
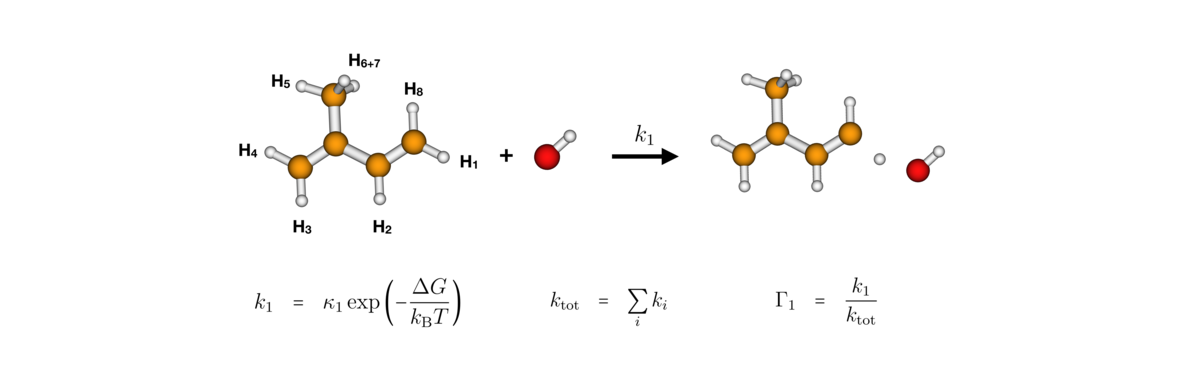
Recent publications:
J. Xue, F. Ma, J. Elm, J. Chen, and H-B. Xie, Atmos. Chem. Phys., 2022, 22, 11543-11555.
Z. Fu, H-B. Xie, J. Elm, Y. Liu, Z. Fu and J. Chen, Environ. Sci. Technol. 2022, 56, 11, 6944-6955.
Z. Fu, HB. Xie, J. Elm, X. Guo, Z. Fu and J. Chen, Environ. Sci. Technol., 2020, 54, 12, 7136–7145.
C. Liu, F. Ma, J. Elm, Z. Fu, W. Tang, J. Chen and H-B. Xie, Chemosphere, 2019, 124411.
The mechanism for how new particles are formed in the atmosphere persists remains the largest uncertainty in models. Thus there is a crucial need to improve the current models with more viable quantum chemical instead of empirical parameterizations. We continuously work on constructing such datasets, to improve the current description of atmospheric transport models.
Recent publications:
We have continuous collaboration with both local (Merete Bilde and Marianne Glasius) and international experimentalists. We primarily model chamber and flow tube processes.
Chemical reactions in the atmosphere is often partitioned into two separate categories: The isolated gas-phase and the particle bulk phase. Very little is known about reactions occurring at the interface between these two extreme, i.e. chemical reactions at the surface of small particles/clusters.
Aerosol particles can grow via uptake of vapour molecules. However, the exact mechanism for the uptake processes remain to a large extent unknown. Some organic compounds have been shown to exhibit a strong interaction with aerosol phase ions, which leads to a "salting in" effect, where the solubility of the compound is increased with increasing ionic strength.
Recent publications:
The scattering of solar radiation by aerosol particles residing in the atmosphere leads to a net cooling effect on the global climate. Aerosol particles can also absorb sunlight if they consists of black carbon (such as soot) or contains a large fraction of light absorbing organic compounds (usually designated brown carbon). Absorption of sunlight will lead to a net heating effect on the global climate. We investigate the Rayleigh light scatting and absorption properties of clusters/particles using response theory methods.